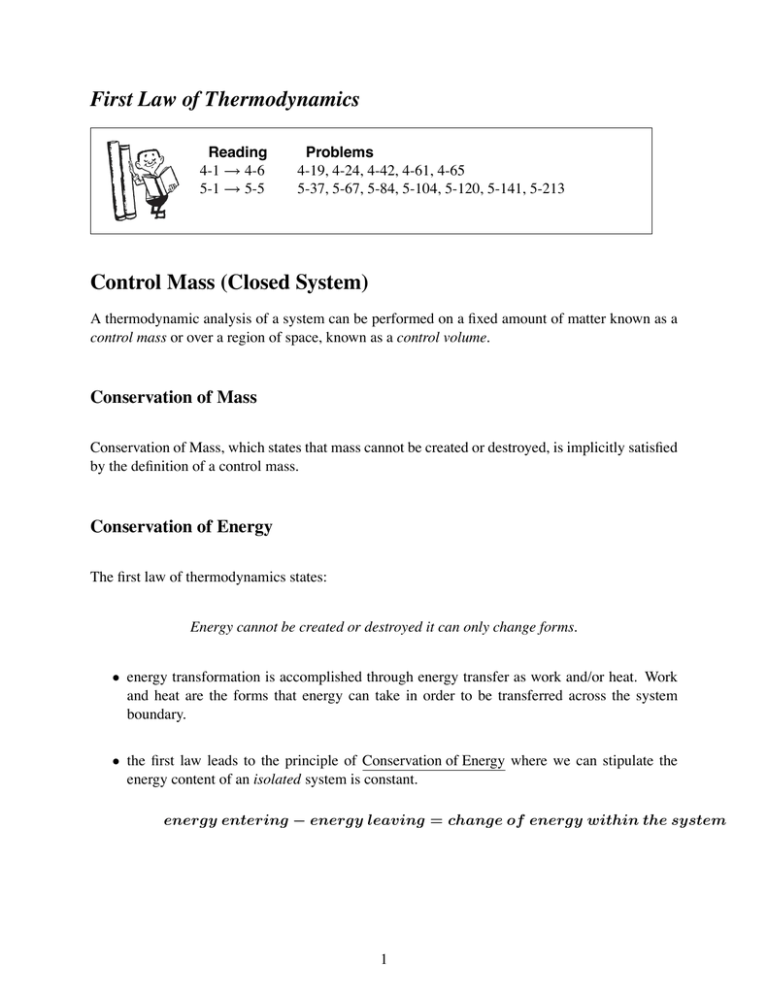
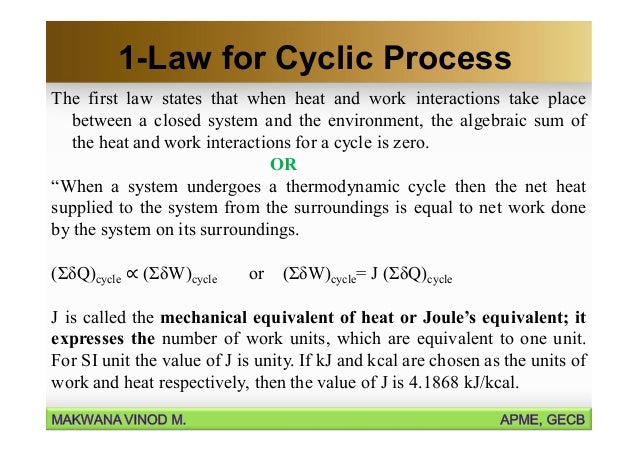
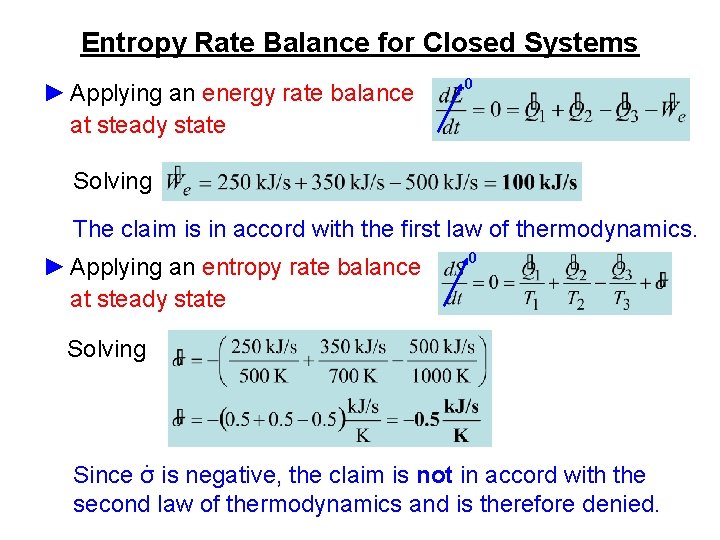
First Law Of Thermodynamics For A Closed System
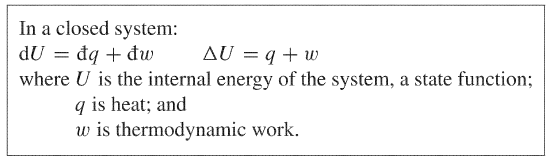
First law of thermodynamics for a closed system. This is first law of thermodynamics for a closed system. Internal Energy is Conserved U 0 For an Isolated System U q w For a Closed System The change in internal energy U of a closed system is equal to the sum of the heat q added to it and the work w done upon it The internal energy of an isolated system is constant The change in internal energy. False Ans - A 7.
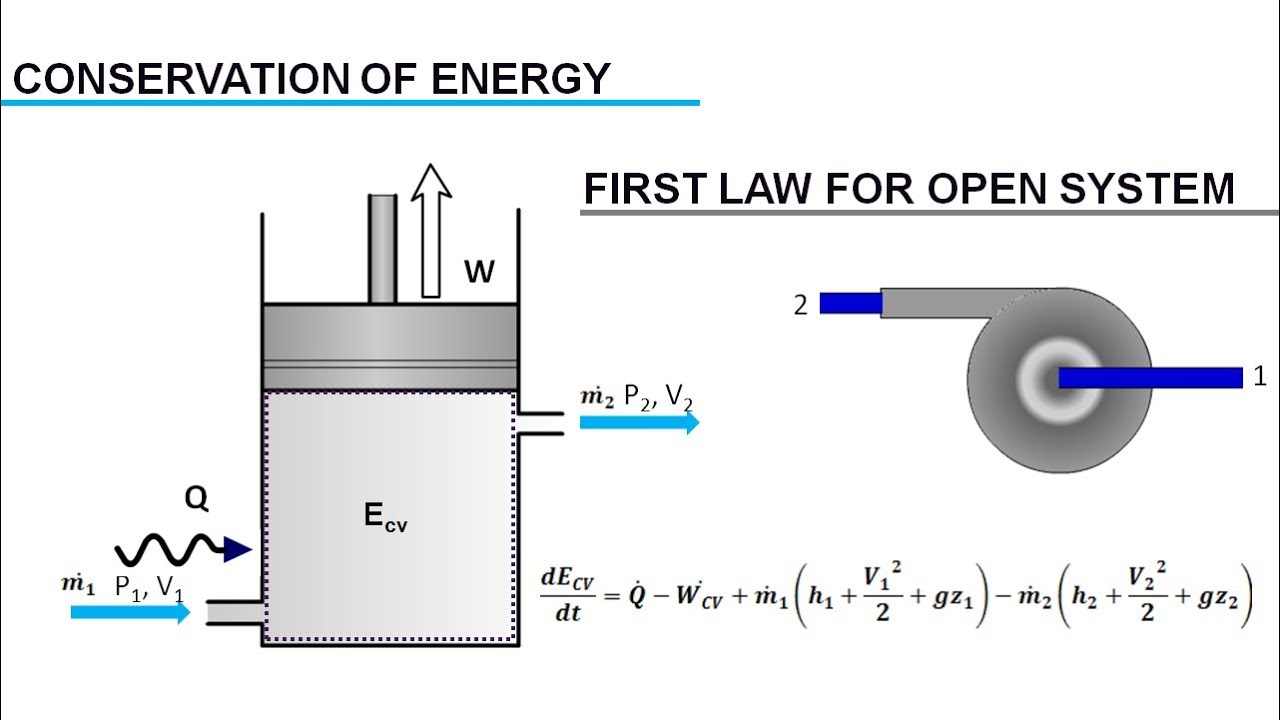
First law of thermodynamics is defined. The rate of energy transfer is kgs kJ kg kJ s k g s k J k g k J s or kW k W. We will briefly consider the three mechanisms for heat transfer.
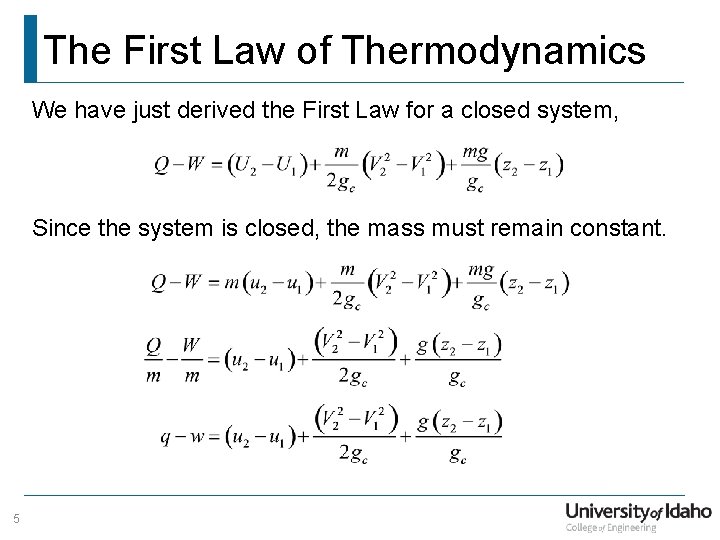
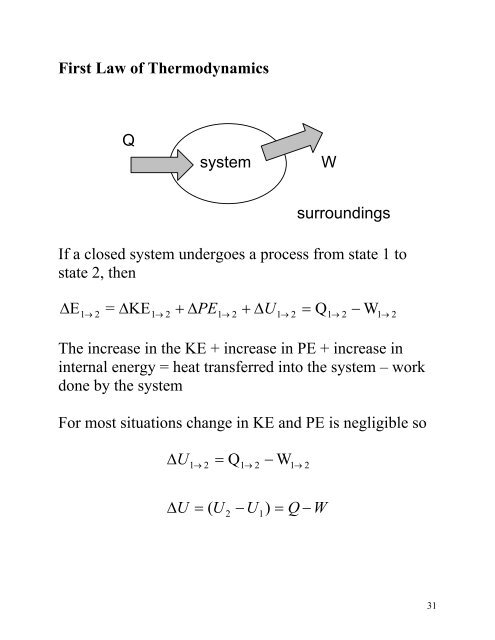
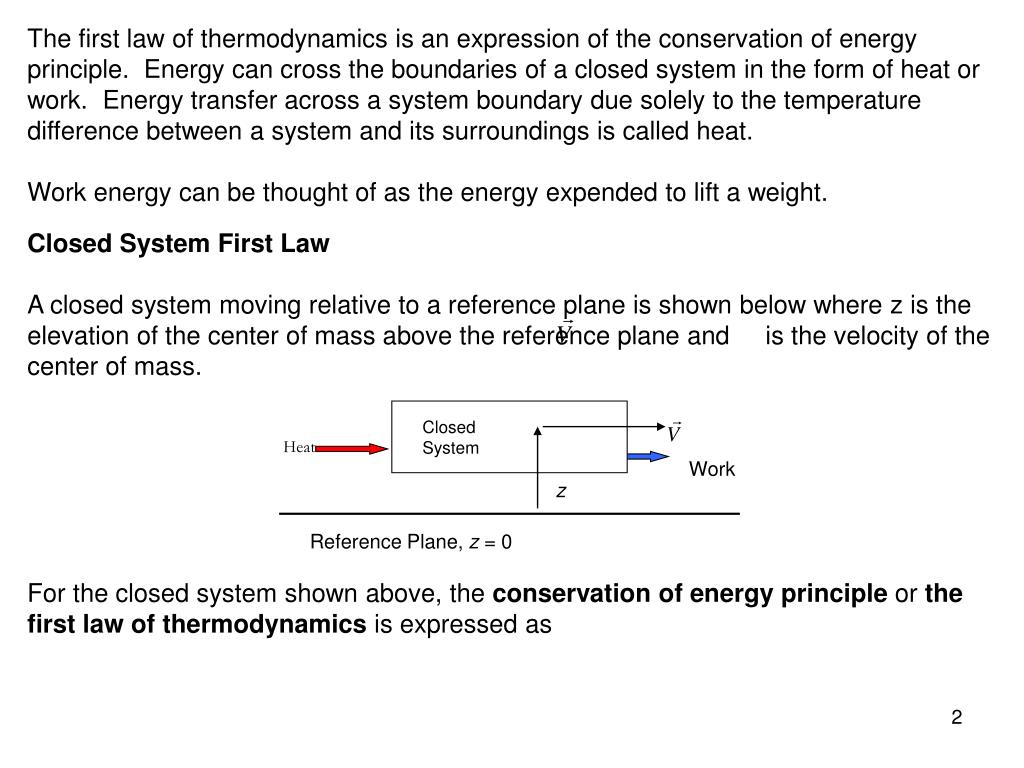
The first law of thermodynamics states. Now the conservation of energy principle or the first law of thermodynamics for closed systems is written as QW U KE PEnet net If the system does not move with a velocity and has no change in elevation the conservation of energy equation reduces to QW Unet net. So there are 2 energy interactions to the gas it will increase by a quantity Q because it is absorbing energy.
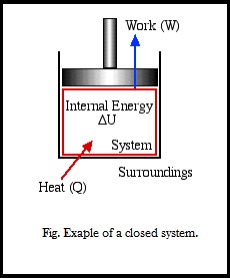
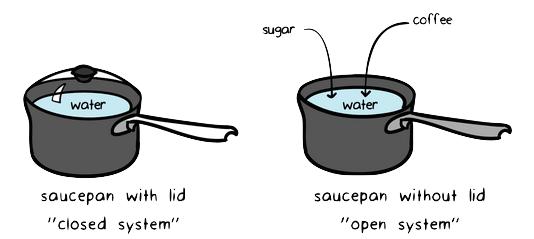
Laws of Thermodynamics in Closed Systems According to the first law of thermodynamics for these systems the change in internal energy is equal to the added work and heat. Thus energy is transferred between the system and the surroundings in the form of heat and work resulting in a change of internal energy of the system. During an interaction between a system and its surroundings the amount of energy gained by the system must be precisely equal to the amount of energy lost by the surroundings.
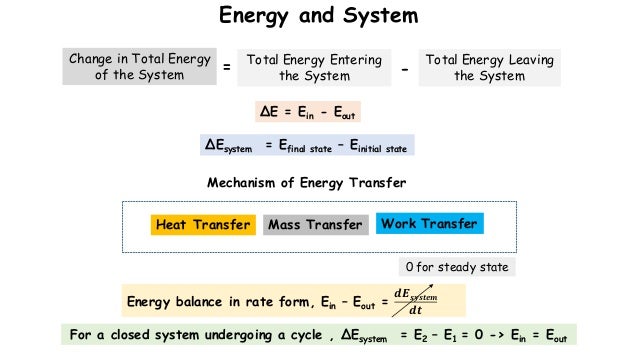
For a closed system in equilibrium KE PE and other kinds of stored energy are zero. The first law states that the rate at which energy flows IN must equal the rate at which energy flows OUT of the system. First Law Of Thermodynamics For A Closed System.
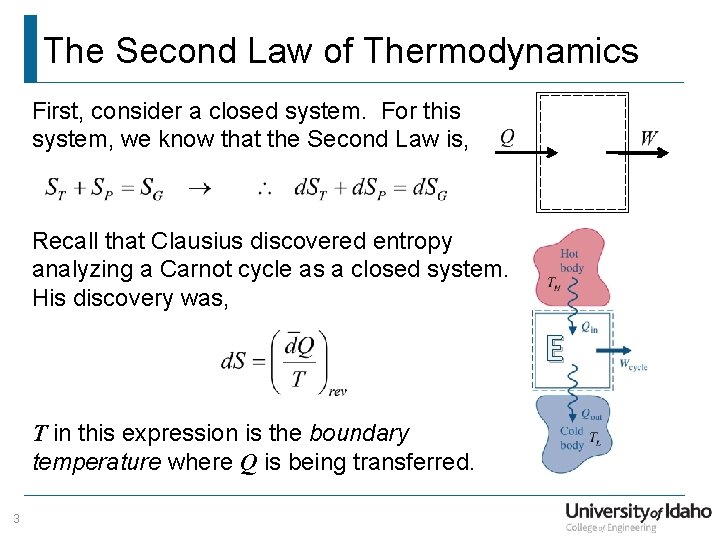
The first law is basically the law of conservation modified for thermodynamics. A thermodynamic system in an equilibrium state possesses a state variable known as the internal energyE. However the first law of thermodynamics states that energy can be converted from one form to another.
These Multiple choice questions and Answers can be attempted by. Thus we consider the law of conservation of energy.
First Law Of Thermodynamics For A Closed System.
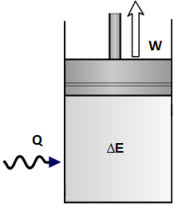
The first law of thermodynamics can be simply stated as follows. What is the First Law of Thermodynamics. We consider the First Law of Thermodynamics applied to stationary closed systems as a conservation of energy principle. And it will decrease by a quantity W since it is losing energy by doing some work. We will briefly consider the three mechanisms for heat transfer. During an interaction between a system and its surroundings the amount of energy gained by the system must be precisely equal to the amount of energy lost by the surroundings. Q dE - W B. This first law can be expressed mathematically as. Collected from the entire web and summarized to include only the most important parts of it.
First law of thermodynamics. ΔE Q - W. Work is said to be done when a. First Law of Thermodynamics for a Closed System. In this video I explained First Law Of Thermodynamics For A Closed System Undergoing A Change Of State Chapter. We consider the First Law of Thermodynamics applied to stationary closed systems as a conservation of energy principle. First law of thermodynamics.


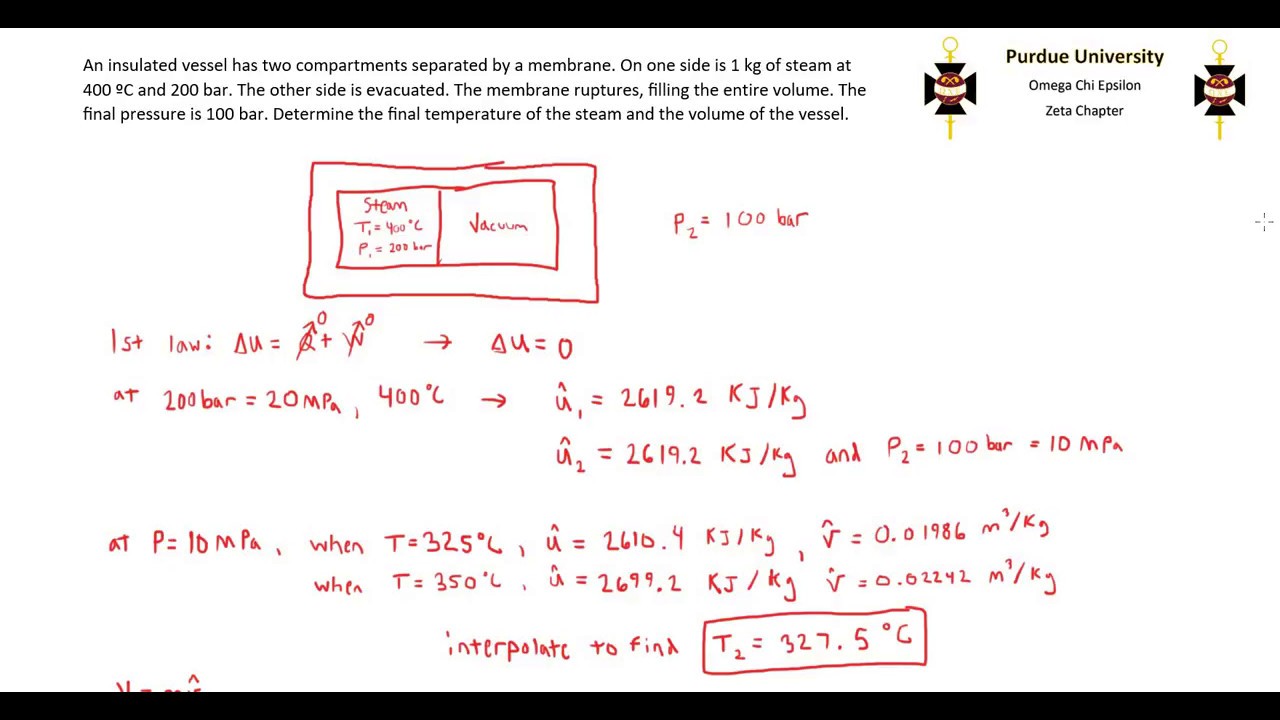


























Post a Comment for "First Law Of Thermodynamics For A Closed System"